COVID Testing Kits

COVID Testing Kits
- (1)
- (6)
- (1)
- (1)
- (1)
- (1)
- (5)
- (1)
- (1)
- (1)
- (2)
- (4)
- (38)
- (1)
- (1)
- (1)
- (6)
- (1)
- (3)
- (1)
- (1)
- (6)
- (1)
- (5)
- (1)
- (2)
- (15)
- (4)
- (5)
- (2)
- (8)
- (2)
- (1)
- (7)
- (21)
- (16)
- (2)
- (7)
- (1)
- (6)
- (3)
- (8)
- (6)
- (31)
- (1)
Filtered Search Results
Abbott BinaxNOW™ COVID-19 Ag Card
Easy to use 15 min. rapid antigen test for detecting active infections of COVID-19

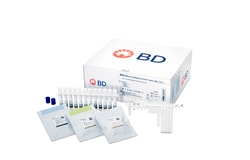
BD Veritor™ System for Rapid Detection of SARS-CoV-2
The BD Veritor™ System for Rapid Detection of SARS-CoV-2 is a chromatographic immunoassay for the direct and qualitative detection of SARS-CoV-2 antigens in nasal swabs from patients with signs and symptoms who are suspected of COVID-19.

BD Veritor™ SARS-CoV-2 Control Swabs
10 SARS-CoV-2 positive and 10 SARS-CoV-2 negative quality control swabs

Applied Biosystems™ TaqMan™ SARS-CoV-2 Control Dilution Buffer
Applied Biosystems TaqMan SARS-CoV-2 Control Dilution Buffer is used to dilute the TaqMan SARS-CoV-2 RNA Control (Cat. No. 956118), which is used with the TaqMan SARS-CoV-2 Pooling Assay Kit (Cat. No. A50790).


Non-distribution item offered as a customer accommodation; additional freight charges may apply.
Learn More
Quidel QuickVue SARS Antigen Test Kit 25/Kit
 Small and Specialty Supplier Partner
Small and Specialty Supplier Partner
Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More

Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More
Detects SARS-CoV-2 antigens directly from anterior nares swab specimens. The QuickVue SARS Antigen Test provides accurate and reliable results in 10 minutes.

Abbott™ ID NOW™ COVID-19 2.0 24 Test Kit
The ID NOW COVID-19 2.0 24 Test Kit helps clinicians to make faster decisions with diagnostic results in real time.

Quidel Sofia™ 2 SARS Antigen Fluorescent Immunoassay (FIA)
 Small and Specialty Supplier Partner
Small and Specialty Supplier Partner
Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More

Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More
Uses immunofluorescence-based lateral flow technology in a sandwich design for qualitative detection of nucleocapsid protein from SARS-CoV-2. Provides automated and objective results in 15 minutes, allowing for testing of patients suspected of COVID-19/2019-nCoV in near-patient testing environments.

| Includes | Cassettes, Reagent tubes, Reagent solution, Swabs, Pipettes (sufficient for 25 tests); Control swabs 1-pos 1-neg |
|---|---|
| Description | Test Kit |
| For Use With (Equipment) | For use with Quidel Sofia 2 Analyzer, Cat. No. 20299. |
| Quantity | 25 Tests/Pk. or 300 Tests/Cs. |
| Clia Complexity | Waived |
| Test Time | 15 min. |
Quidel™ QuickVue™ At-Home OTC COVID-19 Self-Test
 Small and Specialty Supplier Partner
Small and Specialty Supplier Partner
Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More

Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More
The Quickvue™ At-Home OTC COVID-19 test can accurately detect the Omicron variant (among others) of the Sars-CoV-2, the potentially deadly virus that can lead to COVID-19.

| Includes | Individually wrapped sterile foam swabs, test strips, single-use strips, pre-filled tubes, tube holder, instruction sheet |
|---|---|
| Type | At-home COVID Test Kit |
| Format | Kit |
| Sample Type | Nasal Swab |
| Detectable Analytes | SARS-CoV-2 |
| Clia Complexity | Waived (FDA, EUA) |
| Test Time | 10 mins. |
Roche Diagnostics cobas Liat SARS-CoV-2 + Influenza A/B Control Kit
The cobas SARS-CoV-2 & Influenza A/B nucleic acid test is a multiplex real-time polymerase chain reaction (PCR) test that detects and differentiates SARS-CoV-2, Influenza A, and Influenza B. This control kit is for running a control test/lot validation for the assay.

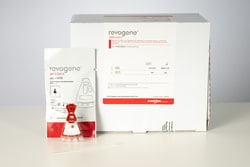
Meridian Bioscience Revogene™ SARS-CoV-2
Detects SARS-CoV-2 RNA from nasopharyngeal, oropharyngeal, anterior nasal, and mid-turbinate swab samples.

SARS-CoV-2 Reagents for BD MAX™ System1
A real time RT-PCR test for the qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal, anterior nasal, mid-turbinate, and oropharyngeal swab specimens, as well as nasopharyngeal wash/aspirate or nasal aspirates obtained from individuals suspected of COVID-19 by their healthcare provider.
Abbott ID NOW™ Rapid Molecular Instrument and ID NOW™ Rapid Molecular COVID-19 Test Kits PROMO
Promotional Kit that contains one ID NOW™ Rapid Molecular Instrument and seven ID NOW™ Rapid Molecular COVID-19 2.0 Test Kits.
BD MAX™ Respiratory Viral Panel (RVP)
This Respiratory Viral Panel is able to detect and differentiate SARS-CoV2, influenza A, influenza B, and RSV.
Quidel™ Lyra SARS-CoV-2 Molecular Assay
 Small and Specialty Supplier Partner
Small and Specialty Supplier Partner
Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More

Small and/or specialty supplier based on Federal laws and SBA requirements.
Learn More
For in vitro qualitative detection of human coronavirus SARS-CoV-2 from viral RNA extracted from nasal, nasopharyngeal (NP) or oropharyngeal (OP) swab specimens from patients with signs and symptoms of COVID-19, Assay targets non-structural Polyprotein (pp1ab) of SARS-CoV-2 virus
| Content And Storage | 2°C to 8°C |
|---|---|
| DoA Calibrators | No |
| Packaging Type | Kit |
| Detectable Analytes | SARS-CoV-2 |
| Quantity | 96 Tests/Kit |
| CE Marker | Yes |
| For Use With (Application) | RT-PCR |
| Clia Complexity | High |
| Test Time | <75 min. after extraction |
| Includes | Tests and controls |
| Specificity | NPA – 92/92 = 100% (95% CI: 95.99 to 100%) |
| Type | Molecular Testing |
| Sensitivity | PPA – 92/92 = 100% (95% CI: 95.99 to 100%) |
| Control Sets | Included |
| For Use With (Equipment) | Emergency Use Authorization (EUA) for use on bioMerieux NucliSENS™ easyMAG™ system or EMAG system, followed by RT-PCR on one of six thermocyclers: Applied Biosystems™ 7500 Fast Dx, Applied Biosystems 7500 Standard, QuantStudioTM Real-Time PCR Instrument, Roche LightCycler™ 480, BioRad CFX96 Touch, or Qiagen Rotor-Gene Q |
| Shelf Life | 6 Months from DOM |
| Certifications/Compliance | Emergency Use Authorization (EUA) |